Nov 14, 2025 | Meet RISK-HUNT3R, News
The first scientific publication introducing the Alternative Safety Profiling Algorithm (ASPA) has been released in ALTEX (October 2025)!
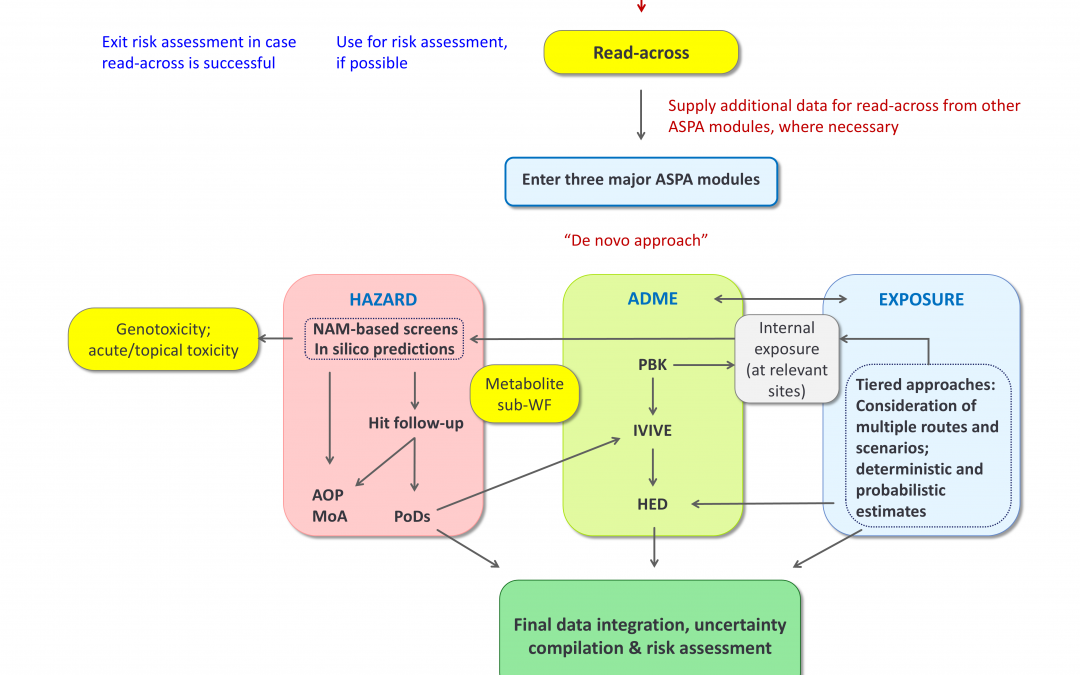
“An Alternative Safety Profiling Algorithm (ASPA) to Transform Next Generation Risk Assessment into a Structured and Transparent Process” presents a broad-purpose, transparent and reproducible risk assessment workflow, designed to make Next Generation Risk Assessment (NGRA) more structured, traceable and applicable in regulatory contexts.
Developed through collaboration within the ASPIS cluster (RISK-HUNT3R, ONTOX, and PrecisionTox), ASPA integrates data on hazard, ADME, and exposure, and is supported by a user-friendly software, ASPA-assist.
This publication marks an important milestone in advancing animal-free, data-driven approaches to chemical safety assessment, and a big step towards the practical implementation of NGRA.
Leist M, Tangianu S, Affourtit F, Braakhuis H, Colbourne J, Cöllen E, Dreser N, Escher SE, Gardner I, Hahn S, Hardy B, Herzler M, Islam B, Kamp H, Magel V, Marx-Stoelting P, Moné MJ, Lundquist P, Ottenbros I, Ouedraogo G, Pallocca G, van de Water B, Vinken M, White A, Pastor M, Luijten M. An Alternative Safety Profiling Algorithm (ASPA) to transform next generation risk assessment into a structured and transparent process. ALTEX. 2025 Oct 16. doi: 10.14573/altex.2509081. Epub ahead of print. PMID: 41099509. https://www.altex.org/index.php/altex/article/view/3041

Sep 24, 2025 | Meet RISK-HUNT3R, News
Last week marked an inspiring milestone for the ASPIS cluster, with two major events taking place back-to-back: EUROTOX 2025 and the ASPIS Open Symposium. Together, they brought scientists, regulators, and industry representatives into one space to share progress, strengthen collaboration, and discuss the future of New Approach Methodologies (NAMs) in regulatory science.
EUROTOX 2025: Bringing science closer to regulatory use
At EUROTOX, RISK-HUNT3R and ASPIS partners showcased advances in NAMs and their role in shaping the future of risk assessment.
• A dedicated session on assessing and communicating uncertainty in Next Generation Risk Assessment (NGRA) sparked valuable discussion on how to make emerging methods more transparent, reliable, and regulatory-ready.
• Another session on advanced human liver test systems highlighted how cutting-edge in vitro models can improve drug and chemical safety evaluation while reducing reliance on animal testing.
These sessions underlined both the scientific potential and the ethical importance of transitioning to modern testing strategies.
ASPIS Open Symposium
Following EUROTOX, the ASPIS Open Symposium brought together RISK-HUNT3R, PrecisionTox, and ONTOX for two days of interactive sessions. Here, the focus was on building momentum around new tools, case studies, and collaborative initiatives to accelerate the regulatory uptake of NAMs.
A particular highlight was the discussion on ASPA, the interactive workflow developed within RISK-HUNT3R to operationalize NGRA, as a key example of how structured workflows can guide users through the NGRA process and support regulatory integration.
Best Poster Award winners for their outstanding contributions:
Eliska Kuchovska (ONTOX; IUF – Leibniz Research Institute for Environmental Medicine) – Regulation of key neurodevelopmental processes by disease pathways and nuclear receptors in a human Neurosphere Assay
Shaleen Glasgow (PrecisionTox; University of Birmingham) – Comparative transcriptomics of Daphnia and Bio-medical models for the evolutionary conservation of a gene network for toxicant associated fatty liver disease
Eike Cöllen (RISK-HUNT3R; University of Konstanz) – Rapid identification of neurotoxicity alerts for multiple compound classes by high-throughput single cell Ca2+ assays
By joining forces, ASPIS projects are moving NAMs from the research bench into regulatory practice, shaping a system that is more reliable, more human-relevant, and less dependent on animal testing.
First ASPA Paper Submitted!
Great news: the very first paper on the ASPA workflow has just been submitted. This is a big milestone for the project and for advancing NGRA in practice.
While under review, you can already explore the preprint here: https://zenodo.org/records/16993943


Jul 23, 2025 | Meet RISK-HUNT3R, News
The whole RISK-HUNT3R consortium convened in June in Helsinki for a truly energizing week, bringing together project members and stakeholders to shape the future of chemical risk assessment.
💡 Across four packed days, we:
- Demonstrated the ASPA workflow for Next Generation Risk Assessment (NGRA) and showcased the NAM toolbox and linked innovations
- Presented ongoing RISK-HUNT3R NGRA case studies
- Engaged in a thought-provoking panel discussion on the impact of RISK-HUNT3R and ASPA for the European Commission NAM Roadmap
- Explored RISK-HUNT3R sustainability efforts and valorisation strategies for NAM-based NGRA
- Held poster session on RISK-HUNT3R scientific and implementation achievements and enjoyed Junior Scientist speed presentations of NAM innovations
- Showcased the submitted OECD IATA case studies
Reflecting on the ASPA and NAMASTOX, our SRAB member Nicholas Ball (Dow) illustratively explained:
“𝘞𝘪𝘵𝘩 𝘢𝘭𝘭 𝘵𝘩𝘦 𝘥𝘪𝘧𝘧𝘦𝘳𝘦𝘯𝘵 𝘤𝘰𝘯𝘯𝘦𝘤𝘵𝘪𝘰𝘯𝘴 𝘣𝘦𝘵𝘸𝘦𝘦𝘯 𝘵𝘩𝘦 𝘥𝘪𝘧𝘧𝘦𝘳𝘦𝘯𝘵 𝘮𝘰𝘥𝘶𝘭𝘦𝘴, 𝘵𝘩𝘦 𝘈𝘚𝘗𝘈 𝘸𝘰𝘳𝘬𝘧𝘭𝘰𝘸 𝘪𝘴 𝘢 𝘭𝘰𝘵 𝘭𝘪𝘬𝘦 𝘢 𝘣𝘰𝘸𝘭 𝘰𝘧 𝘴𝘱𝘢𝘨𝘩𝘦𝘵𝘵𝘪. 𝘓𝘰𝘵𝘴 𝘰𝘧 𝘥𝘪𝘧𝘧𝘦𝘳𝘦𝘯𝘵 𝘴𝘵𝘳𝘢𝘯𝘥𝘴, 𝘧𝘦𝘦𝘥𝘣𝘢𝘤𝘬 𝘭𝘰𝘰𝘱𝘴, 𝘦𝘵𝘤. 𝘕𝘈𝘔𝘈𝘚𝘛𝘖𝘟 𝘢𝘭𝘭o𝘸𝘴 𝘺𝘰𝘶 𝘵𝘰 𝘭𝘰𝘰𝘬 𝘢𝘵 𝘪𝘵 𝘢𝘴 𝘮𝘶𝘤𝘩 𝘤𝘭𝘦𝘢𝘳𝘦𝘳, 𝘴𝘵𝘳𝘢𝘪𝘨𝘩𝘵𝘧𝘰𝘳𝘸𝘢𝘳𝘥 𝘱𝘢𝘵𝘩𝘸𝘢𝘺 – 𝘴𝘰 𝘺𝘰𝘶 𝘰𝘯𝘭𝘺 𝘴𝘦𝘦 𝘵𝘩𝘦 𝘱𝘪𝘦𝘤𝘦 𝘰𝘧 𝘴𝘱𝘢𝘨𝘩𝘦𝘵𝘵𝘪 𝘺𝘰𝘶 𝘯𝘦𝘦𝘥 𝘵𝘰.”
A huge 𝘁𝗵𝗮𝗻𝗸 𝘆𝗼𝘂 to all contributors for making this event a true success. The collaboration, insights and dedication witnessed here will shape the next steps in the journey toward human-relevant, sustainable safety science.

May 27, 2025 | Meet RISK-HUNT3R, News
After months of preparation, the ASPA-NGRA Workshop is finally taking place – 27–28 May at the BfR in Berlin!
Hosted by RISK-HUNT3R and co-organised by EFSA, ECHA, OECD, JRC, EPA, NIH/NIEHS, ONTOX, PrecisionTox, and BfR, it will bring together international experts and stakeholders to critically assess the ASPA workflow for NGRA, ensuring it aligns with the emerging needs of regulatory acceptance for chemical safety in next-generation risk assessment (NGRA).
ASPA is the interactive NGRA workflow that guides users through all steps and decisions of a risk assessment process. It aims to drive consistency and reproducibility in decision-making and is designed to support regulatory and industry stakeholders in the transition towards a safer, animal-free hazard and risk assessment framework.
🔗 Learn more about ASPA
We look forward to inspiring discussion, collaboration, and forward-thinking progress.
Stay updated for the workshop outcome!

Apr 14, 2025 | Meet RISK-HUNT3R, News
The RISK-HUNT3R project is very happy to invite you to the third edition of its public Stakeholder Symposium, taking place on 17-18 June 2025 in Helsinki, Finland. The Symposium will provide an overview of the ASPA NGRA workflow and linked case studies, as well as the developed integrative NAM toolbox, to outline the current scientific state of the art in next-generation risk assessment (NGRA) and what is needed.
This event follows the 3rd Conference on the Roadmap Towards Phasing Out Animal Testing for Chemical Safety Assessments, organised by the European Commission on 16-17 June 2025 in Helsinki.

Feb 28, 2025 | Meet RISK-HUNT3R, News
The RISK-HUNT3R consortium meeting in Egmond aan Zee, Netherlands on 10-13 February 2025 fostered engaging discussions and provided insightful feedback from our Scientific and Regulatory Advisory Board members. Their contributions have helped refine the project’s case studies and further shape our interactive NGRA workflow – ASPA.
With renewed commitment to collaboration and progress, we look forward to the ASPA-NGRA workshop in May, where we will engage with a wide network of experts to further develop ASPA and explore its application across a wide range of chemicals risk assessment.
See you next time in Helsinki!







